肉桂腈
外觀
| 肉桂腈 | |
|---|---|
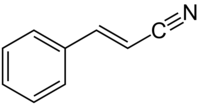
| |
| IUPAC名 3-Phenylprop-2-enenitrile | |
| 識別 | |
| CAS號 | 1885-38-7(反式) 24840-05-9(順氏) 4360-47-8 |
| PubChem | 1550846 |
| ChemSpider | 1267328 |
| SMILES |
|
| InChI |
|
| InChIKey | ZWKNLRXFUTWSOY-QPJJXVBHSA-N |
| EINECS | 217-552-5 |
| 性質 | |
| 化學式 | C9H7N |
| 摩爾質量 | 129.16 g·mol−1 |
| 密度 | 1.0374 g·cm−3 (15.2 °C,反式)[1] |
| 熔點 | 22 °C(295 K)(反式)[2] −10 °C(263 K)(順式)[3] |
| 沸點 | 263.8 °C(537.0 K)(反式)[2] |
| 危險性 | |
GHS危險性符號 
| |
| GHS提示詞 | 危險 |
| H-術語 | H301, H312, H317 |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
肉桂腈是一種有機化合物,化學式為C9H7N。反式肉桂腈可由碘苯和丙烯腈在碳酸鉀存在下、乙酸鈀催化下反應得到,[4]或由反式-β-溴苯乙烯和亞鐵氰酸鉀在鹼存在下、鈀配合物催化下反應得到;[5]順式異構體則可由順式-β-溴苯乙烯在相同反應條件下反應得到。[5]反式異構體在(–)-核黃素存在下於乙腈中光照(402 nm),也能轉化為順式異構體。[6]
反應[編輯]
肉桂腈在鈀催化下加氫,可以得到苯丙腈。[7]它和NMO在四氧化鋨催化下反應,可以得到α,β-二羥基苯丙腈。[8]
參考文獻[編輯]
- ^ Houben, J.; Pfankuch, E. Imidolactones and salts of unsaturated nitriles. Berichte der Deutschen Chemischen Gesellschaft [Abteilung] B: Abhandlungen, 1926. 59B: 1594-1605.
- ^ 2.0 2.1 "PhysProp" data were obtained from Syracuse Research Corporation of Syracuse, New York (US). Retrieved from SciFinder. [2022-06-25].
- ^ William H. Brown, George F. Wright. The Methoxymercurials from cis and trans Styryl Cyanide 1. Journal of the American Chemical Society. 1940-08, 62 (8): 1991–1994 [2022-06-25]. ISSN 0002-7863. doi:10.1021/ja01865a023. (原始內容存檔於2022-06-25) (英語).
- ^ Vivek Srivastava. Carbene Based Palladium-catalyzed Mizoroki-Heck Reaction. Oriental Journal Of Chemistry. 2012-12-22, 28 (4): 1859–1863 [2022-06-25]. ISSN 0970-020X. doi:10.13005/ojc/280444. (原始內容存檔於2018-06-02) (英語).
- ^ 5.0 5.1 Oleg M. Nikitin, Olga V. Polyakova, Petr K. Sazonov, Alexander V. Yakimansky, Mikhail Ya. Goikhman, Irina V. Podeshvo, Tatiana V. Magdesieva. Polymer biquinolyl-containing complexes of Pd(II) as efficient catalysts for cyanation of aryl and vinyl halides with K4Fe(CN)6. New Journal of Chemistry. 2016, 40 (12): 10465–10473 [2022-06-25]. ISSN 1144-0546. doi:10.1039/C6NJ02345B (英語).
- ^ Jan B. Metternich, Denis G. Artiukhin, Mareike C. Holland, Maximilian von Bremen-Kühne, Johannes Neugebauer, Ryan Gilmour. Photocatalytic E → Z Isomerization of Polarized Alkenes Inspired by the Visual Cycle: Mechanistic Dichotomy and Origin of Selectivity. The Journal of Organic Chemistry. 2017-10-06, 82 (19): 9955–9977 [2022-06-25]. ISSN 0022-3263. doi:10.1021/acs.joc.7b01281. (原始內容存檔於2022-06-25) (英語).
- ^ Anuja Nagendiran, Henrik Sörensen, Magnus J. Johansson, Cheuk-Wai Tai, Jan-E. Bäckvall. Nanopalladium-catalyzed conjugate reduction of Michael acceptors – application in flow. Green Chemistry. 2016, 18 (9): 2632–2637 [2022-06-25]. ISSN 1463-9262. doi:10.1039/C5GC02920A (英語).
- ^ Philippe Dupau, Robert Epple, Allen??A. Thomas, Valery??V. Fokin, K.??Barry Sharpless. Osmium-Catalyzed Dihydroxylation of Olefins in Acidic Media: Old Process, New Tricks. Advanced Synthesis & Catalyis. 2002-06, 344 (3-4): 421–433 [2022-06-25]. doi:10.1002/1615-4169(200206)344:3/43.0.CO;2-F.
