槲皮素
| 檞皮素 | |
|---|---|
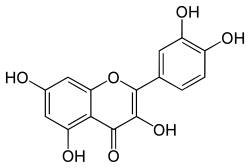
| |
| IUPAC名 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one | |
| 别名 | 栎精 五羟黄酮 檞黄酮 |
| 识别 | |
| CAS号 | 117-39-5 |
| PubChem | 5280343 |
| ChemSpider | 4444051 |
| SMILES |
|
| InChI |
|
| InChIKey | REFJWTPEDVJJIY-UHFFFAOYAW |
| KEGG | C00389 |
| 性质 | |
| 化学式 | C15H10O7 |
| 摩尔质量 | 302.236 g·mol⁻¹ |
| 密度 | 1.799 g/cm3 |
| 熔点 | 316 °C |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
檞皮素(quercetin)又称五羟黄酮、檞黄酮,又称栎精[1],是一种植物性黄酮醇,属于多酚中的黄酮类化合物,存在于水果、蔬菜和谷物等植物中。
檞皮素广泛存在于自然界中。其英文名“quercetin”最早出现于1857年,其来源于“quercetum”,意为栎树林。[2][3]其是一种天然的生长素极性运输抑制剂。[4]
檞皮素含量丰富的食品包括:茶叶(茶树;2000-2500 mg/kg),刺山柑(1800 mg/kg)[5],欧当归(1700 mg/kg),苹果(44 mg/kg),红洋葱(1910 mg/kg,最外层的含量最高[6]),红葡萄,柑橘、蕃茄、花椰菜及其他绿叶蔬菜。此外还有许多浆果的含量也较高,包括覆盆子、欧洲越橘(158 mg/kg,鲜重),越橘(种植74 mg/kg,野生146 mg/kg),蔓越莓(种植83 mg/kg,野生121 mg/kg),沙棘(62 mg/kg),岩高兰(种植53 mg/kg,野生56 mg/kg)[7]及仙人掌的果实。2007年一项研究发现,有机种植的番茄檞皮素含量比传统种植的高出79%。[8]
澳大利亚昆士兰大学的一项研究表明,部分品种的蜂蜜中也存在檞皮素,包括来源于桉树及澳洲茶树的蜂蜜。[9][10]。
苷元
[编辑]檞皮素是许多其他类黄酮苷的苷元。槲皮素与鼠李糖结合形成檞皮苷;与芸香糖结合形成芦丁;与阿拉伯糖结合形成番石榴苷;与乳糖结合形成金丝桃苷。
生理活性
[编辑]对大鼠生物利用度的研究显示,当放射性同位素标记的槲皮素-4-葡萄糖苷通过胃肠道后,其被转化为酚酸。[11]
檞皮素既尚未被科学的证明其具有任何疗效,也没有得到任何监管机构的批准。美国食品与药品管理局尚未批准任何关于槲皮素的功效说明。[12]不过达沙替尼和槲皮素的混合物也一种潜在的返老药(Senolytic),一项人体初步临床试验显示,达沙替尼和槲皮素的混合物在患有糖尿病肾脏病变的人类患者中,确实会降低部分组织当中衰老细胞的数量。[13]
炎症
[编辑]一些实验室的研究表明槲皮素可能具有抗炎特性[14][15],并在研究其潜在的疗效。[15][16]
檞皮素可减轻花粉热的症状。[17]其一种酶改性衍生物被发现具有减轻花粉热眼部症状的作用。[18][19][20]
一项对老鼠的研究表明,槲皮素能有效的减少速释型烟酸引起的潮红现象,部分途径为减少前列腺素D2的产生。[21]一个四人的试验性临床试验给出的初步数据支持该观点。[22]
癌症
[编辑]实验室体外细胞研究显示,檞皮素也可转变为致癌物,但这项研究并没有报告其会增加动物或人类的患癌风险。[23][24][25]
美国癌症协会说道,虽然檞皮素“已被选为对许多包括癌症的疾病有效的物质”,并且“一些早期的实验结果显示其具有开发前景,但现在还没有可靠的临床证据说明檞皮素可以预防或治疗人类癌症。”充足的水果和蔬菜的摄入可能降低患癌症的风险[26],槲皮素是许多可能的作用源之一受到研究。
在动物实验中,檞皮素被推测有可能降低患某些癌症的风险。[27][28]一项时长8年的研究发现,三种黄酮类化合物——山柰酚、檞皮素和杨梅素——可降低吸烟者患胰腺癌的风险。[29]
通过檞皮素与超声波结合,可抑制体外培养的皮肤癌和前列腺癌细胞。[30]
代谢
[编辑]檞皮素已被证明可增加大鼠的能量代谢,但仅限于短期(短于8周)。[14]檞皮素对小鼠运动耐受性的影响与增加线粒体生物合成有关。[15]小鼠口服12.5至25 mg/kg浓度依次增加的檞皮素,可增加线粒体生物标志物的基因表达,并可改善运动耐受性。.[31]
已有有关于檞皮素对结节病、哮喘、肥胖与糖尿病的葡萄糖吸收的安全性和有效性的初步研究。[32]
也有学者声称檞皮素可降低高血压患者的血压[33],及可降低肥胖者的低密度脂蛋白胆固醇的水平。[34]
体外研究表明檞皮素和白藜芦醇联合应用可抑制脂肪细胞的产生。[35]
药物相互作用
[编辑]檞皮素有一些抗生素配伍禁忌;其可能影响氟喹诺酮的作用,因檞皮素也具有竞争结合DNA旋转酶的能力。尚未确定其是否能抑制或增强氟奎诺酮的效果。[36]
《AHFS药物信息》(2010年)[37]将檞皮素标记为CYP2C8的抑制剂,并具体的说明其与紫杉醇可能形成有害的相互作用。由于紫杉醇由CYP2C8代谢,其生物利用度可能增加或不可预测,可能导致毒副作用。[38][39]
此外,檞皮素还被描述为CYP2C9的抑制剂[40],及CYP3A4的抑制剂[41]和诱导剂[42]。CYP2C9和CPY3A4都是细胞色素P450混合功能氧化酶系统的组分,因此这些酶参与外来物质的代谢。
参考文献
[编辑]- ^ 存档副本. [2020-07-02]. (原始内容存档于2020-07-25).
- ^ Quercetin. Merriam-Webster. [2011-06-30]. (原始内容存档于2020-09-24).
- ^ Quercitin (biochemistry). Encyclopedia Brittanica. [2011-06-30]. (原始内容存档于2014-03-18).
- ^ Christiane Fischer, Volker Speth, Sonja Fleig-Eberenz, and Gunther Neuhaus. lnduction of Zygotic Polyembryos in Wheat: lnfluence of Auxin Polar Transport (PDF). Plant Cell. 1999-10, 9 (10): 1767–1780. PMC 157020
 . PMID 12237347. doi:10.1105/tpc.9.10.1767.
. PMID 12237347. doi:10.1105/tpc.9.10.1767.
- ^ USDA Database for the Flavonoid Content of Selected Foods 互联网档案馆的存档,存档日期2012-07-16.
- ^ Crystal Smith, Kevin A. Lombard, Ellen B. Peffley, Weixin Liu. Genetic Analysis of Quercetin in Onion (Allium cepa L.) Lady Raider (PDF). The Texas Journal of Agriculture and Natural Resource (Agriculture Consortium of Texas). 2003, 16: 24–8. (原始内容 (PDF)存档于2007年2月25日).
- ^ Sari H. Häkkinen; et al. Content of the Flavonols Quercetin, Myricetin, and Kaempferol in 25 Edible Berries. Journal of Agricultural and Food Chemistry. 1999, 47 (6): 2274–9. PMID 10794622. doi:10.1021/jf9811065.
- ^ A. E. Mitchell, Y. J. Hong, E. Koh, D. M. Barrett, D. E. Bryant, R. F. Denison and S. Kaffka. Ten-Year Comparison of the Influence of Organic and Conventional Crop Management Practices on the Content of Flavonoids in Tomatoes. Journal of Agricultural and Food Chemistry. 2007, 55 (15): 6154–9. PMID 17590007. doi:10.1021/jf070344.
- ^ Honey Research Unit
- ^ honey fingerprinting. [2011-06-30]. (原始内容存档于2016-03-04).
- ^ Mullen W; et al. Bioavailability of [2-(14)C]quercetin-4'-glucoside in rats. J Agric Food Chem. December 2008, 2456 (24): 12127–37. PMID 19053221. doi:10.1021/jf802754s.
- ^ US FDA, Center for Food Safety and Nutrition, Qualified Health Claims Subject to Enforcement Discretion, April 2007 存档副本. [2011-06-30]. (原始内容存档于2009-05-14).
- ^ Hickson, LaTonya J.; Langhi Prata, Larissa G.P.; Bobart, Shane A.; Evans, Tamara K.; Giorgadze, Nino; Hashmi, Shahrukh K.; Herrmann, Sandra M.; Jensen, Michael D.; Jia, Qingyi; Jordan, Kyra L.; Kellogg, Todd A.; Khosla, Sundeep; Koerber, Daniel M.; Lagnado, Anthony B.; Lawson, Donna K.; LeBrasseur, Nathan K.; Lerman, Lilach O.; McDonald, Kathleen M.; McKenzie, Travis J.; Passos, João F.; Pignolo, Robert J.; Pirtskhalava, Tamar; Saadiq, Ishran M.; Schaefer, Kalli K.; Textor, Stephen C.; Victorelli, Stella G.; Volkman, Tammie L.; Xue, Ailing; Wentworth, Mark A.; Wissler Gerdes, Erin O.; Zhu, Yi; Tchkonia, Tamara; Kirkland, James L. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. September 2019, 47: 446–456. PMC 6796530
 . PMID 31542391. doi:10.1016/j.ebiom.2019.08.069.
. PMID 31542391. doi:10.1016/j.ebiom.2019.08.069.
- ^ 14.0 14.1 Laura K. Stewart, Jeff L. Soileau, David Ribnicky, Zhong Q. Wang, Ilya Raskin, Alexander Poulev, Martin Majewski, William T. Cefalu, and Thomas W. Gettys. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism. 2008, 57.
- ^ 15.0 15.1 15.2 J. Mark Davis, E. Angela Murphy, Martin D. Carmichael, and Ben Davis, Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance, Am J Physiol Regul Integr Comp Physiol, 2009, 296
- ^ Phys Ed: Is Quercetin Really a Wonder Sports Supplement? (页面存档备份,存于互联网档案馆)By Gretchen Reynolds. New York Times, October 7, 2009. Review of the research.
- ^ Balabolkin, II; Gordeeva, GF; Fuseva, ED; Dzhunelov, AB; Kalugina, OL; Khamidova, MM. Use of vitamins in allergic illnesses in children. Voprosy meditsinskoi khimii. 1992, 38 (5): 36–40. PMID 1492394.
- ^ Hirano, T; Kawai, M; Arimitsu, J; Ogawa, M; Kuwahara, Y; Hagihara, K; Shima, Y; Narazaki, M; Ogata, A. Preventative effect of a flavonoid, enzymatically modified isoquercitrin on ocular symptoms of Japanese cedar pollinosis. Allergology international : official journal of the Japanese Society of Allergology. 2009, 58 (3): 373–82. PMID 19454839. doi:10.2332/allergolint.08-OA-0070.
- ^ Kawai, M; Hirano, T; Arimitsu, J; Higa, S; Kuwahara, Y; Hagihara, K; Shima, Y; Narazaki, M; Ogata, A. Effect of enzymatically modified isoquercitrin, a flavonoid, on symptoms of Japanese cedar pollinosis: a randomized double-blind placebo-controlled trial. International archives of allergy and immunology. 2009, 149 (4): 359–68. PMID 19295240. doi:10.1159/000205582.
- ^ Mainardi, T; Kapoor, S; Bielory, L. Complementary and alternative medicine: herbs, phytochemicals and vitamins and their immunologic effects. The Journal of allergy and clinical immunology. 2009, 123 (2): 283–94; quiz 295–6. PMID 19203652. doi:10.1016/j.jaci.2008.12.023.
- ^ Papaliodis D, Boucher W, Kempuraj D, Theoharides TC. The flavonoid luteolin inhibits niacin-induced flush. Brit J Pharmacol. 2008, 153 (7): 1382–87. PMC 2437911
 . PMID 18223672. doi:10.1038/sj.bjp.0707668.
. PMID 18223672. doi:10.1038/sj.bjp.0707668.
- ^ Kalogeromitros, D; Makris, M; Chliva, C; Aggelides, X; Kempuraj, D; Theoharides, TC. A quercetin containing supplement reduces niacin-induced flush in humans. International journal of immunopathology and pharmacology. 2008, 21 (3): 509–14. PMID 18831918.
- ^ Verschoyle RD, Steward WP, Gescher AJ. Putative cancer chemopreventive agents of dietary origin-how safe are they?. Nutr Cancer. 2007, 59 (2): 152–62. PMID 18001209. doi:10.1080/01635580701458186 (不活跃 2009-06-26).
- ^ Rietjens IM, Boersma MG, van der Woude H, Jeurissen SM, Schutte ME, Alink GM. Flavonoids and alkenylbenzenes: mechanisms of mutagenic action and carcinogenic risk. Mutat. Res. July 2005, 574 (1–2): 124–38. PMID 15914212. doi:10.1016/j.mrfmmm.2005.01.028.
- ^ van der Woude H, Alink GM, van Rossum BE; et al. Formation of transient covalent protein and DNA adducts by quercetin in cells with and without oxidative enzyme activity. Chem. Res. Toxicol. December 2005, 18 (12): 1907–16. PMID 16359181. doi:10.1021/tx050201m.
- ^ Guidance for Industry: A Food Labeling Guide XI. Appendix C: Health Claims, 21 CFR 101.76 and 21 CFR 101.78, April 2008. US Department of Health and Human Services, Food and Drug Administration. [2011-06-30]. (原始内容存档于2013-03-07).
- ^ Neuhouser ML. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer. 2004, 50 (1): 1–7. PMID 15572291. doi:10.1207/s15327914nc5001_1.
- ^ Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. October 2008, 269 (2): 315–25. PMID 18467024. doi:10.1016/j.canlet.2008.03.046.
- ^ Nöthlings U; et al. Flavonols and pancreatic cancer risk. American Journal of Epidemiology. 2007, 166 (8): 924–931. PMID 17690219. doi:10.1093/aje/kwm172.
- ^ Paliwal S; Sundaram, J; Mitragotri, S. Induction of cancer-specific cytotoxicity towards human prostate and skin cells using quercetin and ultrasound. British Journal of Cancer. 2005, 92 (3): 499–502 [2011-06-30]. PMC 2362095
 . PMID 15685239. doi:10.1038/sj.bjc.6602364. (原始内容存档于2017-08-16).
. PMID 15685239. doi:10.1038/sj.bjc.6602364. (原始内容存档于2017-08-16).
- ^ Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009, 296 (4): R1071–7. PMID 19211721. doi:10.1152/ajpregu.90925.2008.
- ^ Clinicaltrials.gov, National Institutes of Health. [2011-06-30]. (原始内容存档于2020-07-25).
- ^ Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 1 November 2007, 137 (11): 2405–11. PMID 17951477.
- ^ Egert S; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009, 102 (7): 1065–1074. PMID 19402938. doi:10.1017/S0007114509359127.
- ^ Yang JY, Della-Fera MA, Rayalam S, Ambati S, Hartzell DL, Park HJ, Baile CA. Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 2008, 82 (19–20): 1032–9. PMID 18433793. doi:10.1016/j.lfs.2008.03.003.
- ^ Hilliard JJ, Krause HM, Bernstein JI; et al. A comparison of active site binding of 4-quinolones and novel flavone gyrase inhibitors to DNA gyrase. Adv. Exp. Med. Biol. 1995, 390: 59–69. PMID 8718602.
- ^ 存档副本. [2011-06-22]. (原始内容存档于2011-07-07).
- ^ Bun SS, Ciccolini J, Bun H, Aubert C, Catalin J. Drug interactions of paclitaxel metabolism in human liver microsomes. J Chemother. 2003-06, 15 (3): 266–74. PMID 12868554.
- ^ Bun SS, Giacometti S, Fanciullino R, Ciccolini J, Bun H, Aubert C. Effect of several compounds on biliary excretion of paclitaxel and its metabolites in guinea-pigs. Anticancer Drugs. 2005–07, 16 (6): 675–82. PMID 15930897. doi:10.1097/00001813-200507000-00013.
- ^ Si Dayong, Wang Y, Zhou Y-H, Guo Y, Wang J, Zhou H, Li Z-S, Fawcett JP. Mechanism of CYP2C9 inhibition by flavones and flavonols (PDF). Drug Metabolism and Disposition. March 2009, 37 (3): 629–634. [2011-06-30]. PMID 19074529. doi:10.1124/dmd.108.023416. (原始内容存档 (PDF)于2008-12-17).
- ^ Su-Lan Hsiu; Yu-Chi Hou; Yao-Horng Wang; Chih-Wan Tsao; Sheng-Fang Sue; and Pei-Dawn L. Chao. Quercetin significantly decreased cyclosporin oral bioavailability in pigs and rats. Life Sciences. 6 December 2002, 72 (3): 227–235. PMID 12427482. doi:10.1016/S0024-3205(02)02235-X.
- ^ Judy L. Raucy. Regulation of CYP3A4 Expression in Human Hepatocytes by Pharmaceuticals and Natural Products. Drug Metabolism and Disposition. 1 May 2003, 31 (3): 533–539. PMID 12695340. doi:10.1124/dmd.31.5.533.
