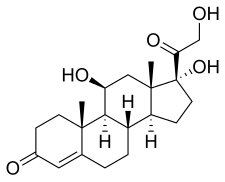
氫羥腎上腺皮質素
外觀
 | |
 | |
| 臨床資料 | |
|---|---|
| 商品名 | A-hydrocort, Cortef, Solu-cortef, others[1] |
| 其他名稱 | Cortisol; 11β,17α,21-Trihydroxypregn-4-ene-3,20-dione; 11β,17α,21-Trihydroxyprogesterone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682206 |
| 核准狀況 | |
| 懷孕分級 | |
| 給藥途徑 | 口服給藥 (片劑), 靜脈注射, 外塗, 肛門給藥 |
| 藥物類別 | 皮質類固醇; 糖皮質素; 礦物皮質素 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 | |
| 藥物動力學數據 | |
| 生物利用度 | 口服: 96 ± 20%[10][11] |
| 血漿蛋白結合率 | 92 ± 2% (92–93%)[10][11] |
| 藥物代謝 | 11β-HSDs, 其它[11] |
| 代謝產物 | 可體松, 其它[11] |
| 藥效起始時間 | 口服: 1.2 ± 0.4 hours (Tmax)[10] |
| 生物半衰期 | 1.2–2.0 小時[10][11] |
| 作用時間 | 8–12 小時[12] |
| 識別資訊 | |
| |
| CAS號 | 50-23-7 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| 化學資訊 | |
| 化學式 | C21H30O5 |
| 摩爾質量 | 362.47 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
氫羥腎上腺皮質素(英語:Hydrocortisone),又稱氫化皮質酮或氫化可體松,是皮質醇作為藥物的名稱[13]。它是一種糖皮質激素,具有抗炎和免疫抑制作用。可以治療:腎上腺機能不全、腎上腺生殖器綜合症、高血鈣、甲狀腺炎、類風濕性關節炎、皮膚炎、哮喘和慢性阻塞性肺病(COPD)等疾病[1]。它是治療腎上腺機能不全的首選藥物[14]。此藥可以口服、局部塗抹或注射給藥[1]。在長期使用而要停藥時,應逐漸減少後才能完全停用[1]。
它的副作用包括情緒改變、增加感染風險和水腫等[1]。長期使用後,常見副作用包括骨質疏鬆、胃部不適、身體虛弱、容易瘀青和念珠菌感染[1]。雖然可以在懷孕期使用,但安全性仍未確認[15]。
此藥於 1936 年獲得專利,並於 1941 年獲准用於治療[16] [17]。並列入世界衛生組織基本藥物標準清單[18],市面上有多家學名藥[1]。2014年發展中國家每日口服藥物的每日費用約0.27美元[19]。在美國,一個月的治療費用通常不到 25美元[20]。 2017 年,它是美國最常用處方藥的第 154 名,開立的處方超過 400 萬張[21] [22]。
參考資料
[編輯]- ^ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Hydrocortisone. Drugs.com. American Society of Health-System Pharmacists. February 9, 2015 [30 August 2016]. (原始內容存檔於20 September 2016).
- ^ Prescribing medicines in pregnancy database. Therapeutic Goods Administration (TGA). [21 February 2021]. (原始內容存檔於20 December 2016).
- ^ Hydrocortisone Notice of enforcement policy (PDF). FDA. [31 December 2022]. (原始內容存檔 (PDF)於12 March 2023).
- ^ Ala-cort- hydrocortisone cream. DailyMed. [21 February 2021]. (原始內容存檔於27 October 2020).
- ^ Ala-scalp- hydrocortisone lotion. DailyMed. [21 February 2021]. (原始內容存檔於21 April 2021).
- ^ Alkindi Sprinkle- hydrocortisone granule. DailyMed. [21 February 2021]. (原始內容存檔於10 February 2022).
- ^ Anusol HC- hydrocortisone acetate suppository. DailyMed. [21 February 2021]. (原始內容存檔於10 February 2022).
- ^ Cortef- hydrocortisone tablet. DailyMed. [21 February 2021]. (原始內容存檔於17 April 2021).
- ^ Efmody EPAR. European Medicines Agency (EMA). 24 March 2021 [14 June 2021]. (原始內容存檔於14 June 2021). Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ 10.0 10.1 10.2 10.3 Czock D, Keller F, Rasche FM, Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clinical Pharmacokinetics. 2005, 44 (1): 61–98. PMID 15634032. S2CID 24458998. doi:10.2165/00003088-200544010-00003.
- ^ 11.0 11.1 11.2 11.3 11.4 Lennernäs H, Skrtic S, Johannsson G. Replacement therapy of oral hydrocortisone in adrenal insufficiency: the influence of gastrointestinal factors. Expert Opinion on Drug Metabolism & Toxicology. June 2008, 4 (6): 749–758. PMID 18611115. S2CID 73248541. doi:10.1517/17425255.4.6.749.
- ^ Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, Brown JP, Cohen A, Kim H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. August 2013, 9 (1): 30. PMC 3765115
 . PMID 23947590. doi:10.1186/1710-1492-9-30
. PMID 23947590. doi:10.1186/1710-1492-9-30  .
.
- ^ Becker, Kenneth L. Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. 2001: 762. ISBN 9780781717502. (原始內容存檔於2016-09-14) (英語).
- ^ Hamilton, Richart. Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. 2015: 202. ISBN 9781284057560.
- ^ Hydrocortisone Pregnancy and Breastfeeding Warnings. Drugs.com. [1 September 2016]. (原始內容存檔於20 September 2016).
- ^ 美國專利第2,183,589號
- ^ Fischer, Jnos; Ganellin, C. Robin. Analogue-based Drug Discovery. John Wiley & Sons. 2006: 484 [2019-03-01]. ISBN 9783527607495. (原始內容存檔於2019-03-02) (英語).
- ^ WHO Model List of Essential Medicines (19th List) (PDF). World Health Organization. April 2015 [8 December 2016]. (原始內容存檔 (PDF)於13 December 2016).
- ^ Hydrocortisone. International Drug Price Indicator Guide. [1 September 2016]. (原始內容存檔於2018-08-27).
- ^ Hamilton, Richart. Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. 2015: 202. ISBN 9781284057560.
- ^ The Top 300 of 2020. ClinCalc. [11 April 2020]. (原始內容存檔於12 February 2021).
- ^ Hydrocortisone - Drug Usage Statistics. ClinCalc. [11 April 2020]. (原始內容存檔於12 April 2020).
